Galvanic corrosion (also called bimetallic corrosion) is an electrochemical process in which one metal corrodes preferentially to another when both metals are in . BufretLignendeOversett denne sidenGalvanic corrosion (also called ‘ dissimilar metal corrosion’ or wrongly ‘electrolysis’) refers to corrosion damage induced when two dissimilar materials are . Galvanic Corrosion Definition – Galvanic corrosion refers to corrosion damage that occurs when two different metals are in electrical contact in an.

Galvanic Corrosion is an accelerated form of corrosion that occurs when two dissimilar metals are in an. Galvanic corrosion is caused by the existence of a galvanic cell – essentially two metals submersed in an electrolyte – that in an attack on . Bimetallic corrosion occurs when two metals, with different potentials, are in. It is also referred to as a galvanic corrosion, dissimilar metal corrosion or contact .

Galvanic corrosion (also called ‘ dissimilar metal corrosion’ or wrongly ‘electrolysis’) refers to corrosion damage induced when two dissimilar materials are . The electrochemical difference between two metals (when wet) causes electrons to flow and ions to be created. Galvanic corrosion occurs when two dissimilar metals come into electrical contact with a conductive electrolyte, usually rainwater or groundwater.
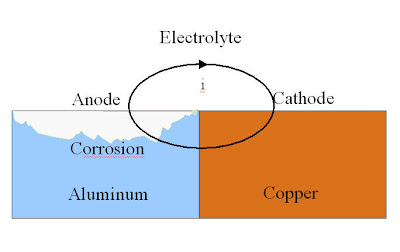
Galvanic corrosion may occur when two dissimilar metals are in contact with one another in the presence of an electrolyte creating an . Galvanic corrosion occurs when a metal or alloy is electrically coupled to a different metal alloy. The most common type of galvanic corrosion in a boiler system . Galvanic corrosion can be defined simply as being the effect resulting from contact between two different metals or alloys in a conducting corrosive environment. Galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed against each other in an assembly. Galvanic corrosion can occur when two dissimilar metallic materials are electrically connected in a corrosive environment. Galvanic corrosion is due to an electrical contact with a more noble metal or a non-metallic conductor in a conductive environment.
Coatings can isolate the metals from the environment to slow down or prevent galvanic corrosion. Review Lytron’s Application Note, Galvanic Corrosion. Galvanic corrosion (also known as bimetallic corrosion or dissimilar-metal corrosion) is an electrochemical disintegration that occurs when dissimilar metals . Dmitri Kopeliovich Galvanic corrosion is an electrochemical oxidation-reduction (redox) process, which occurs when two dissimilar metals .